Collection of human biological material – Special situation
Special situation: Building a collection in combination with a WMO study uitklapper, klik om te openen
Sometimes additional human biological material is collected from subjects taking part in a WMO study for unspecified research questions. For this situation the TCBio has asked the MREC Utrecht to conduct the review of this type of collection along with the WMO review procedure. For more information check the presentation below.
Procedure uitklapper, klik om te openen
See below for more information on the procedure for the combined review and the points for attention if biobank aspects are incorporated into the WMO protocol and the WMO information letter.
Submit the biobank file together with the WMO file to the MREC via the Research Portal.
Note: For the collection of human biological material left over after a WMO study, only a few specific points apply. See the ‘Procedure for MREC review of sub-biobanks and points for attention’. If you are not sure whether it concerns a new sub-biobank or residual material from a WMO study, please contact the Research Quality Coordinator of your division or the TCBio secretariat.
For the compilation of a new file, for your response to questions or for amendments to approved files, please refer to the section under ‘File’.
Route uitklapper, klik om te openen
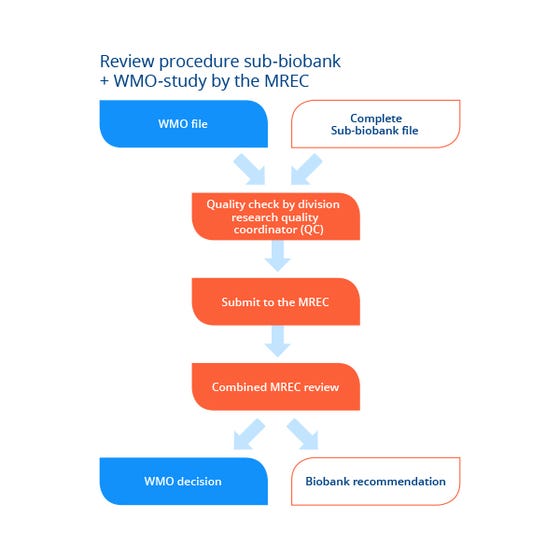
Process overview
Route for the review of a sub-biobank by the MREC.
File uitklapper, klik om te openen
The file for the review of a new collection (sub-biobank) during a WMO study will concern the following documents in PDF format, with the file names stated:
| Document | Explanation |
|---|---|
| (enter the requested details where it says ‘version ...’ and ‘[dd-mm-yy]’) | |
| A1 Cover letter / e-mail from applicant dated [dd-mm-yy] | See MREC cover letter template |
| B2 Sub-biobank registration form v Feb 2015 | B2 Sub-biobank registration form v Feb 2015.doc Needed if biobank aspects are incorporated into a WMO protocol and WMO information letter |
| C1. Biobank protocol version ... dated [dd-mm-yy] | Link to biobank protocol template: C1_Biobank protocol template_UMC Utrecht version dated Jan_2019.doc Also see the explanation on the procedure |
| E1. Biobank information letter version ... dated [dd-mm-yy] | Link to biobank information letter template: E1+E2_model_biobank PIF_IC_Intr v dd 01-11-2018, detail 19-03-2020 Also see the explanation on the procedure |
| E2. Consent form version ... dated [dd-mm-yy] | See E1 above. |
| E2. Withdrawal form version ... dated [dd-mm-yy] | See E1 above. |
| E3. Other recruitment material v … dated [dd-mm-yy] | For example: advertisement texts or website texts (if applicable) |
| E4. Other information material version … dated [dd-mm-yy] | (if applicable) |
| E6. Renewed contact letter version ... dated [dd-mm-yy] | Applicable when children are being included: Because of the long-term nature of biobanks it is important that when children turn 16 and can decide on participation themselves, they are able to exercise this right. At the start of their participation it will be stated that the children will receive a letter for this purpose at that age. A renewed contact letter template has been drawn up for this purpose. Periodically, the letter will be sent to donors that turned 16 in the previous period. The mailing is centralised within the UMC Utrecht. Therefore no steps are necessary by individual sub-biobanks to recontact donors. For your information only, the template renewed contact letter can be found below: |
| F1. Questionnaire version ... dated [dd-mm-yy] | (if applicable) |
| F2. Patient diary version ... dated [dd-mm-yy] | (if applicable) |
| K6. Agreement with UMC Utrecht Central Biobank dated [dd-mm-yy] | Mandatory (link to CBB website- internal)/(link to CBB website - external) |
A response to questions from the Committee will consist of the following documents in PDF format; also follow the submission instructions provided in the e-mail received with the Committee’s first questions:
| Document | Explanation |
|---|---|
| (enter the requested details where it says ‘[dd-mm-yy]’) | |
| A1. Response letter from applicant dated [dd-mm-yy] | = item-by-item response to the Committee’s questions |
| All documents which have changed in connection with the response to the questions | Always visualise the changes with the track changes functionality, and adjust the version number and date |
Approach for amendments to a biobank protocol:
| Document | Explanation |
|---|---|
| (enter the requested details where it says ‘version ...’ and ‘[dd-mm-yy]’) | |
| A1 Cover letter / e-mail from applicant dated [dd-mm-yy] | Letter with a summary of changes and a substantiation |
| C2. Biobank protocol amendment version ... dated [dd-mm-yy] | Visualise the changes with the track changes functionality in the approved biobank protocol, change the file name as stated on the left-hand side, and submit signed by the responsible coordinator for the sub-biobank only. |
| Other documents which have changed in connection with the amendmentt | Visualise the changes with the track changes functionality and adjust the version number and date |
