Collection of human biological material
A positive recommendation from the reviewing committee and approval from the Executive Board are needed for the collection of:
- human biological material for unspecified research questions
- additional human biological material obtained for unspecified research questions from subjects taking part in a WMO study
Collections are also referred to as sub-biobanks, even if no human biological material is being stored.
Review uitklapper, klik om te openen
The TCBio reviews the collection of human biological material based on the criteria of UMC Utrecht’s Biobank Regulations. For the compilation of a new file, for your response to questions or for amendments to approved files, please refer to the section under ‘File’ below.
Route uitklapper, klik om te openen
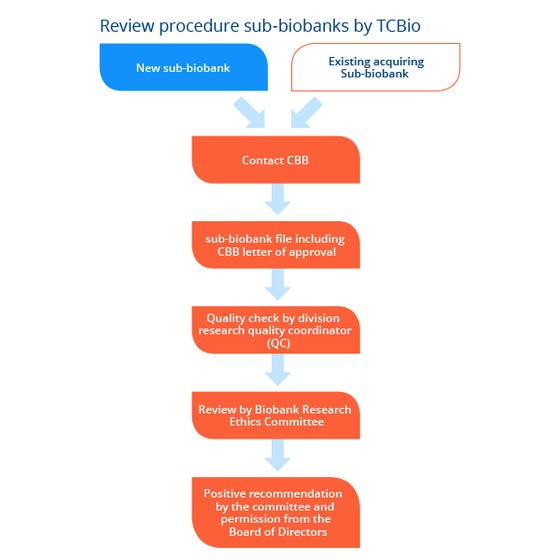
Process overview
Route for the review of a sub-biobank by the TCBio.
File uitklapper, klik om te openen
The file for the review of a new collection (sub-biobank) will consist of the following documents in PDF format, with the file names stated:
| Document | Explanation |
|---|---|
| (enter the requested details where it says ‘version ...’ and ‘[dd-mm-yy]’) | |
| A1 Cover letter / e-mail from applicant dated [dd-mm-yy] | A separate cover letter with explanatory notes is optional when submitting a file. Any explanation can also be provided in the cover e-mail. |
| C1. Biobank protocol version ... dated [dd-mm-yy] | Link to biobank protocol template: C1_Biobank protocol template_UMC Utrecht version dated Jan_2019.doc |
| E1. Biobank information letter version ... dated [dd-mm-yy] | Link to biobank information letter template: E1+E2_Biobank PIF template_IC_Intr v dated 01 November 2018, detail 19 March 2020 |
| E2. Consent form version ... dated [dd-mm-yy] | See E1 above. |
| E2. Withdrawal form version ... dated [dd-mm-yy] | See E1 above. |
| E3. Other recruitment material v … dated [dd-mm-yy] | For example: advertisement texts or website texts (if applicable) |
| E4. Other information material version … dated [dd-mm-yy] | (if applicable) |
| E6. Renewed contact letter version ... dated [dd-mm-yy] | Applicable when children are being included: Because of the long-term nature of biobanks it is important that when children turn 16 and can decide on participation themselves, they are able to exercise this right. At the start of their participation it will be stated that the children will receive a letter for this purpose at that age. A renewed contact letter template has been drawn up for this purpose. Periodically, the letter will be sent to donors that turned 16 in the previous period. The mailing is centralised within the UMC Utrecht. Therefore no steps are necessary by individual sub-biobanks to recontact donors. For your information only, the template renewed contact letter can be found below: |
| F1. Questionnaire version ... dated [dd-mm-yy] | (if applicable) |
| F2. Patient diary version ... dated [dd-mm-yy] | (if applicable) |
| K6. Agreement with UMC Utrecht Central Biobank dated [dd-mm-yy] | Mandatory (link to CBB - internal)/(link to CBB - external) |
A response to questions from the Committee will consist of the following documents in PDF format; also follow the submission instructions provided in the e-mail received with the Committee’s questions:
| Document | Explanation |
|---|---|
| (enter the requested details where it says ‘[dd-mm-yy]’) | |
| A1. Response letter from applicant dated [dd-mm-yy] | = item-by-item response to the Committee’s questions |
| All documents which have changed in connection with the response to the questions | In amended documents, always visualise the changes with the track changes functionality, and adjust the version number and date |
Approach for amendments to a biobank protocol:
| Document | Explanation |
|---|---|
| (enter the requested details where it says ‘version ...’ and ‘[dd-mm-yy]’) | |
| A1 Cover letter / e-mail from applicant dated [dd-mm-yy] | Letter with a summary of changes and a substantiation |
| C2. Biobank protocol amendment version ... dated [dd-mm-yy] | Visualise the changes with the track changes functionality in the approved biobank protocol, change the file name as stated on the left-hand side, and submit signed by the responsible coordinator for the sub-biobank only. |
| Other documents which have changed in connection with the amendment | Visualise the changes with the track changes functionality and adjust the version number and date |
