Biobanks UMC Utrecht
The rules for establishing a sub-biobank and use of human biological material for scientific research are described in the UMC Utrecht Biobank Regulations.pdf. Prior to either activity, approval needs to be obtained from the Biobank Research Ethics Committee.
The most important aspects from the Regulations and an overview of the review procedures are summarized in the presentation below.
Biobank system UMC Utrecht uitklapper, klik om te openen
Position of TCBio in the biobank system
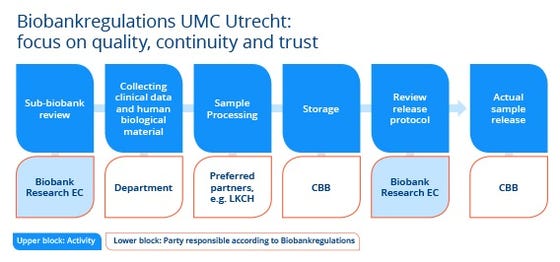
Background Biobanks uitklapper, klik om te openen
UMC Utrecht Biobanks, consisting of human biological material and associated data are increasingly important for medical scientific research. Typical for a biobank is that the specific research question for which the material will be used is only globally known at the time donors make their material available to the biobank. Also, in most cases the researcher does not know for which specific purpose the material will be used and by whom. At the time of donation to the biobank, the donor actually gives away part of his control rights over the material to the biobank. To ensure support for biobanks now and in the future, donors have to be able to trust that their material and data will be handled in a proper manner in the biobank and during the medical scientific research. The following aspects are important for the trust of donors:
1) protection of confidentiality of the material and the data,
2) manner of consent of the donor,
3) return of results,
4) ownership of the material,
5) commercial use.
In addition, donors have to be able to trust that their material and data will only be used for relevant scientific research.
The issues above have been worked out in the UMC Utrecht Biobank Regulations.pdf that have been in effect since 2013. With the Biobank Regulations the UMC Utrecht aims to build a high quality infrastructure for medical scientific research for all UMC Utrecht researchers and her partners. To reach this goal, an independent committee, the Biobank Research Ethics Committee (TCBio), reviews if the human biological material and data are collected and stored in a responsible way in the biobank according to the criteria of the Biobank Regulations. Similarly, the committee reviews if the material and associated data will be used in a responsible way in scientific research. This governance model doesn’t solely serve the interests of the donors but also those of the researcher and society as a whole by making sure that the (scarce) material will be used for the right projects. In addition, the quality and registration of the human biological material in the biobank is guarded by the Central Biobank UMC Utrecht.
